Reneo Pharmaceuticals is rapidly advancing REN001, an investigational clinical-stage compound, as a potential treatment for genetic mitochondrial myopathies, including FAOD and PMM. Currently there are no EMA or FDA approved drugs for treating PMM.
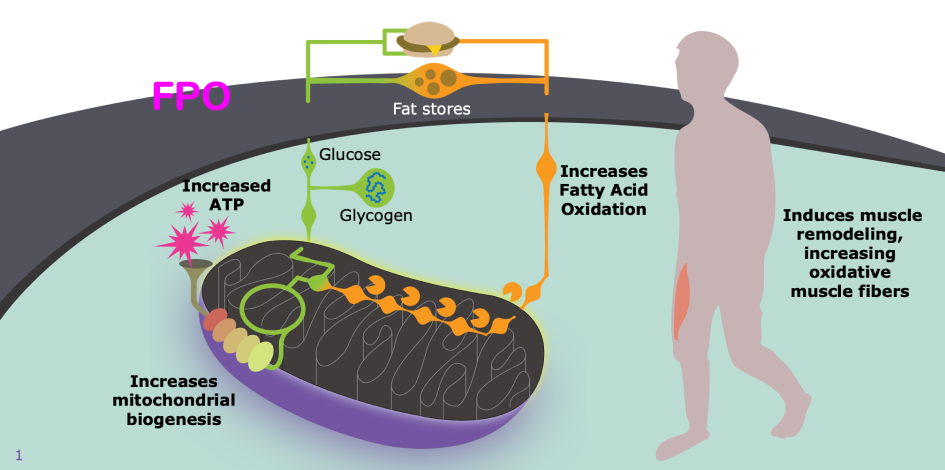
REN001 is an investigational clinical-stage compound known to control a number of genes involved in the mitochondrial activity. Reneo is developing this compound to improve cellular energy metabolism by enhancing mitochondrial function and potentially increasing the number of mitochondria.
Mitochondria are known as the powerhouses of the cell, where carbohydrates, fats and proteins are used to generate the energy the body needs. Mitochondrial dysfunction has been implicated in many disorders that affect skeletal muscle metabolism and impact daily function and quality of life.

Reneo Pharmaceuticals drug development and research experts studied REN001’s background. What they discovered is that REN001 emulates mitochondrial function in the muscle, which could support mitochondrial energy production.
Patients with primary mitochondrial myopathies (PMM), fatty acid oxidation disorders (FAOD), and glycogen storage disorder, could benefit from the investigational drug, specifically by preserving muscle function, preventing muscle injury, weakness and wasting, thereby impacting daily function and quality of life. The next phase clinical trial will start in early 2021.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Duis ut mollis leo. Suspendisse mollis nisl elit, et cursus quam venenatis quis. Aliquam congue tincidunt velit id aliquam. Lorem ipsum dolor sit amet, consectetur adipiscing elit. Phasellus at mattis augue, et ultricies tellus. Quisque et magna quis felis aliquam fermentum in at arcu. Sed pellentesque egestas nibh, et fringilla nisi aliquam ac.
Name | Name | Name | Name | Name |
|---|---|---|---|---|